Pyrrole Red Dab on:
[Wikipedia]
[Google]
[Amazon]
Pyrrole is a
 Pyrrole itself is not naturally occurring, but many of its derivatives are found in a variety of
Pyrrole itself is not naturally occurring, but many of its derivatives are found in a variety of

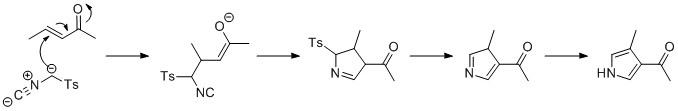
 Pyrroles can be prepared by
Pyrroles can be prepared by 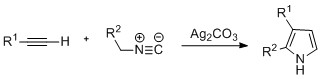

 .
.
 Proline can be used as precursor of aromatic pyrroles in secondary natural products, as in prodigiosins.
Proline can be used as precursor of aromatic pyrroles in secondary natural products, as in prodigiosins.  The biosynthesis of Prodigiosin involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-serine, L-methionine, pyruvate, and 2-octenal.
Ring A is synthesized from L-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD+ to yield pyrrole ring A.
The biosynthesis of Prodigiosin involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-serine, L-methionine, pyruvate, and 2-octenal.
Ring A is synthesized from L-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD+ to yield pyrrole ring A.
 Ring A is then expanded via the polyketide synthase pathway to incorporate L-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of L-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from L-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A–B ring structures.
Ring A is then expanded via the polyketide synthase pathway to incorporate L-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of L-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from L-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A–B ring structures.

 Pyrroles react easily with
Pyrroles react easily with

 Substitution at C3 can be achieved through the use of ''N''-substituted 3-bromopyrrole, which can be synthesized by bromination of ''N''-silylpyrrole with NBS.
Substitution at C3 can be achieved through the use of ''N''-substituted 3-bromopyrrole, which can be synthesized by bromination of ''N''-silylpyrrole with NBS.
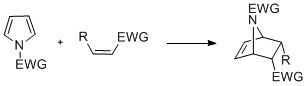 Pyrroles can react with
Pyrroles can react with 
Synthesis of pyrroles (overview of recent methods)
Substitution reaction mechanisms of nitrogen-containing heteroaromatics
{{Authority control
heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
, a five-membered ring with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ...
C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, C4H4NCH3. Porphobilinogen
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll.
The structure of the molecule can be described a ...
, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consisti ...
.
Pyrroles are components of more complex macrocycles, including the porphyrinogen
In biochemistry a porphyrinogen is a member of a class of naturally occurring compounds with a tetrapyrrole core, a macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples in ...
s and products derived therefrom, including porphyrin
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical com ...
s of heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consisti ...
, the chlorin
In organic chemistry, chlorins are tetrapyrrole pigments that are partially hydrogenated porphyrins. The parent chlorin is an unstable compound which undergoes air oxidation to porphine.
The name chlorin derives from chlorophyll. Chlorophylls a ...
s, bacteriochlorins, and chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to a ...
s.
Properties
Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified bydistillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distilla ...
immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
heterocycle, like furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
and thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reacti ...
. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it has chemical shifts at 6.68 (H2, H5) and 6.22 (H3, H4). Pyrrole is an extremely weak base for an amine, with a conjugate acid p''K''a of −3.8. The most thermodynamically stable pyrrolium cation (C4H6N+) is formed by protonation at the 2 position. Substitution of pyrrole with alkyl substituents provides a more basic molecule—for example, tetramethylpyrrole has a conjugate acid p''K''a of +3.7. Pyrrole is also weakly acidic at the N–H position, with a p''K''a of 16.5.
As a hydrogen bonding Lewis acid it is classified as a hard acid and the ECW model lists its acid parameters as ''E''A = 1.38 and ''C''A = 0.68.
History
Pyrrole was first detected by F. F. Runge in 1834, as a constituent ofcoal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasi ...
. In 1857, it was isolated from the pyrolysate of bone. Its name comes from the Greek ''pyrrhos'' (, “reddish, fiery”), from the reaction used to detect it—the red color that it imparts to wood when moistened with hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
.
Occurrence in nature
cofactors
Cofactor may also refer to:
* Cofactor (biochemistry), a substance that needs to be present in addition to an enzyme for a certain reaction to be catalysed
* A domain parameter in elliptic curve cryptography, defined as the ratio between the order ...
and natural products
A natural product is a natural Chemical compound, compound or chemical substance, substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural product ...
. Common naturally produced molecules containing pyrroles include vitamin B12, bile pigments like bilirubin
Bilirubin (BR) (Latin for "red bile") is a red-orange compound that occurs in the normal catabolic pathway that breaks down heme in vertebrates. This catabolism is a necessary process in the body's clearance of waste products that arise from the ...
and biliverdin
Biliverdin (latin for green bile) is a green tetrapyrrolic bile pigment, and is a product of heme catabolism.Boron W, Boulpaep E. Medical Physiology: a cellular and molecular approach, 2005. 984-986. Elsevier Saunders, United States. It is the pi ...
, and the porphyrins
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical compo ...
of heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consisti ...
, chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to a ...
, chlorin
In organic chemistry, chlorins are tetrapyrrole pigments that are partially hydrogenated porphyrins. The parent chlorin is an unstable compound which undergoes air oxidation to porphine.
The name chlorin derives from chlorophyll. Chlorophylls a ...
s, bacteriochlorins, and porphyrinogens. Other pyrrole-containing secondary metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
s include PQQ, makaluvamine M, ryanodine, rhazinilam, lamellarin, prodigiosin, myrmicarin, and sceptrin. The syntheses of pyrrole-containing haemin, synthesized by Hans Fischer
Hans Fischer (; 27 July 1881 – 31 March 1945) was a German organic chemist and the recipient of the 1930 Nobel Prize for Chemistry "for his researches into the constitution of haemin and chlorophyll and especially for his synthesis of haem ...
was recognized by the Nobel Prize.
Pyrrole is a constituent of tobacco smoke and may contribute to its toxic effects.
Synthesis
Pyrrole is prepared industrially by treatment offuran
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
with ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
in the presence of solid acid catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s, like SiO2 and Al2O3.
Pyrrole can also be formed by catalytic dehydrogenation of pyrrolidine.
Laboratory routes
Several syntheses of the pyrrole ring have been described.Hantzsch pyrrole synthesis
The Hantzsch pyrrole synthesis is the reaction of β-ketoesters (1) with ammonia (or primary amines) and α-haloketones (2) to give substituted pyrroles (3).Knorr pyrrole synthesis
The Knorr pyrrole synthesis involves the reaction of an α-amino ketone or an α-amino-β-ketoester with an activated methylene compound. The method involves the reaction of an α-amino
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent s ...
ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
(1) and a compound containing a methylene group
In organic chemistry, a methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon atom, which is connected to the remainder of the molecule by two single bonds. The group may be represented as , where the '< ...
α to (bonded to the next carbon to) a carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
(2).
Paal–Knorr pyrrole synthesis
In the Paal–Knorr pyrrole synthesis, a 1,4-dicarbonyl compound reacts with ammonia or a primary amine to form a substituted pyrrole.Van Leusen reaction
The Van Leusen reaction can be used to form pyrroles, by reaction oftosylmethyl isocyanide
TosMIC (toluenesulfonylmethyl isocyanide) is an organic compound with the formula CH3C6H4SO2CH2NC. The molecule contains both sulfonyl and isocyanide groups. It is a colourless solid that, unlike many isocyanides, is odorless. It is prepared by de ...
(TosMIC) with an enone in the presence of base, in a Michael addition
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...
. A 5-''endo'' cyclization then forms the 5-membered ring, which reacts to eliminate the tosyl group. The last step is tautomerization to the pyrrole.

Barton–Zard synthesis
The Barton–Zard synthesis proceeds in a manner similar to the Van Leusen synthesis. An isocyanoacetate reacts with a nitroalkene in a 1,4-addition, followed by 5-''endo''-''dig'' cyclization, elimination of thenitro group
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nitr ...
, and tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
ization.
Piloty–Robinson pyrrole synthesis
The starting materials in the Piloty–Robinson pyrrole synthesis, named for Gertrude and Robert Robinson andOskar Piloty
Oskar Piloty (30 April 1866 – 6 October 1915) was a German chemist.
Life
Oskar Piloty was born the son of the painter Karl von Piloty in Munich. Due to the closeness of the Piloty family to the chemist Ludwig Knorr, who later married the ...
, are two equivalents of an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
and hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
. The product is a pyrrole with substituents at the 3 and 4 positions. The aldehyde reacts with the diamine to an intermediate di-imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
(R−C=N−N=C−R). In the second step, a ,3sigmatropic rearrangement
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular reaction. The name ''sigmatropic'' is the result of a compounding of the long ...
takes place between. Addition of hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
leads to ring closure and loss of ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
to form the pyrrole. The mechanism
Mechanism may refer to:
* Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission
*Mechanism (biology), explaining how a feature is created
*Mechanism (philosophy), a theory that ...
was developed by the Robinsons.
In one modification, propionaldehyde
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a slightly fruity odour. It is produced on a large scale industrially.
Production
Propionalde ...
is treated first with hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
and then with benzoyl chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula . It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring () with an acyl chloride () substituent. It is ...
at high temperatures and assisted by microwave irradiation Microwave chemistry is the science of applying microwave radiation to chemical reactions. Microwaves act as high frequency electric fields and will generally heat any material containing mobile electric charges, such as polar molecules in a solvent ...
:
Cycloaddition-based routes
Pyrroles bearing multiple substituents are obtained from the reaction ofmünchnone
Münchnone (synonyms: 1,3-oxazolium-5-oxide; 1,3-oxazolium-5-olate; anhydro-5-hydroxy-1,3-oxazolium hydroxide; 5-hydroxy-1,3-oxazolium hydroxide, inner salt; oxido-oxazolium) is a mesoionic heterocyclic aromatic chemical compound, with the molecula ...
s and alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s. The reaction mechanism involves 1,3-dipolar cycloaddition followed by loss of carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
by a retro- Diels–Alder process. Similar reactions can be performed using azalactones.
 Pyrroles can be prepared by
Pyrroles can be prepared by silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
-catalyzed cyclization of alkynes with isonitriles, where R2 is an electron-withdrawing group, and R1 is an alkane, aryl group, or ester. Examples of disubstituted alkynes have also been seen to form the desired pyrrole in considerable yield. The reaction is proposed to proceed via a silver acetylide
In organometallic chemistry, acetylide refers to chemical compounds with the chemical formulas and , where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure (where R is an organic side c ...
intermediate. This method is analogous to the azide–alkyne click chemistry
In chemical synthesis, click chemistry is a class of biocompatible small molecule reactions commonly used in bioconjugation, allowing the joining of substrates of choice with specific biomolecules. Click chemistry is not a single specific reaction ...
used to form azoles.

Other methods
One synthetic route to pyrrole involves thedecarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is t ...
of ammonium mucate, the ammonium salt of mucic acid
Mucic acid, C6H10O8 or HOOC-(CHOH)4-COOH (also known as galactaric or meso-galactaric acid) is an aldaric acid obtained by nitric acid oxidation of galactose or galactose-containing compounds such as lactose, dulcite, quercite, and most varietie ...
. The salt is typically heated in a distillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distilla ...
setup with glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
as a solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
.
Biosynthesis of pyrroles
The de novo biosynthesis of pyrrole rings begins withaminolevulinic acid
δ-Aminolevulinic acid (also dALA, δ-ALA, 5ALA or 5-aminolevulinic acid), an endogenous non-proteinogenic amino acid, is the first compound in the porphyrin synthesis pathway, the pathway that leads to heme in mammals, as well as chlorophyll in p ...
(ALA), which is synthesized from glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinogeni ...
and succinyl-CoA
Succinyl-coenzyme A, abbreviated as succinyl-CoA () or SucCoA, is a thioester of succinic acid and coenzyme A.
Sources
It is an important intermediate in the citric acid cycle, where it is synthesized from α-ketoglutarate by α-ketoglutarate de ...
. ALA dehydratase catalyzes the condensation of two ALA molecules via a Knorr-type ring synthesis to form porphobilinogen
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll.
The structure of the molecule can be described a ...
(PBG). This later reacts to form, for example, the macrocycles heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consisti ...
and chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to a ...
.
 .
.
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the prot ...
is biosynthetically derived from the amino acid L-glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
. Glutamate-5-semialdehyde
Glutamate-5-semialdehyde is a non-proteinogenic amino acid involved in both the biosynthesis and degradation of proline and arginine (via ornithine), as well as in the biosynthesis of antibiotics, such as carbapenems. It is synthesized by the redu ...
is first formed by glutamate 5-kinase
In enzymology, a glutamate 5-kinase () is an enzyme that catalyzes the chemical reaction
:ATP + L-glutamate \rightleftharpoons ADP + L-glutamate 5-phosphate
Thus, the two substrates of this enzyme are ATP and L-glutamate, whereas its two pr ...
(ATP-dependent) and glutamate-5-semialdehyde dehydrogenase
In enzymology, a glutamate-5-semialdehyde dehydrogenase () is an enzyme that catalyzes the chemical reaction
:L-glutamate 5-semialdehyde + phosphate + NADP+ \rightleftharpoons L-glutamyl 5-phosphate + NADPH + H+
The 3 substrates of this enzyme ...
(which requires NADH or NADPH). This can then either spontaneously cyclize to form 1-pyrroline-5-carboxylic acid, which is reduced to proline by pyrroline-5-carboxylate reductase
In enzymology, a pyrroline-5-carboxylate reductase () is an enzyme that catalyzes the chemical reaction
:L-proline + NAD(P)+ \rightleftharpoons 1-pyrroline-5-carboxylate + NAD(P)H + H+
The 3 substrates of this enzyme are L-proline, NAD+, and ...
(using NADH or NADPH), or turned into ornithine
Ornithine is a non-proteinogenic amino acid that plays a role in the urea cycle. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency. The radical is ornithyl.
Role in urea cycle
L-Ornithine is one of the produ ...
by ornithine aminotransferase
Ornithine aminotransferase (OAT) is an enzyme which is encoded in human by the OAT gene located on chromosome 10.
The OAT involved in the ultimate formation of the non-essential amino acid proline from the amino acid ornithine. Ornithine aminot ...
, followed by cyclisation by ornithine cyclodeaminase
The enzyme ornithine cyclodeaminase (EC 4.3.1.12) catalyzes the chemical reaction
L-ornithine \rightleftharpoons L-proline + NH4+
This enzyme belongs to the family of lyases, specifically ammonia lyases, which cleave carbon-nitrogen bonds. Th ...
to form proline.
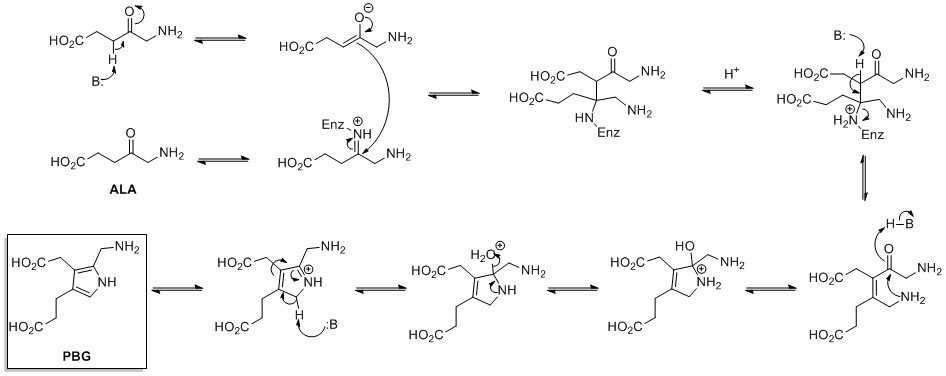
 Proline can be used as precursor of aromatic pyrroles in secondary natural products, as in prodigiosins.
Proline can be used as precursor of aromatic pyrroles in secondary natural products, as in prodigiosins.  The biosynthesis of Prodigiosin involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-serine, L-methionine, pyruvate, and 2-octenal.
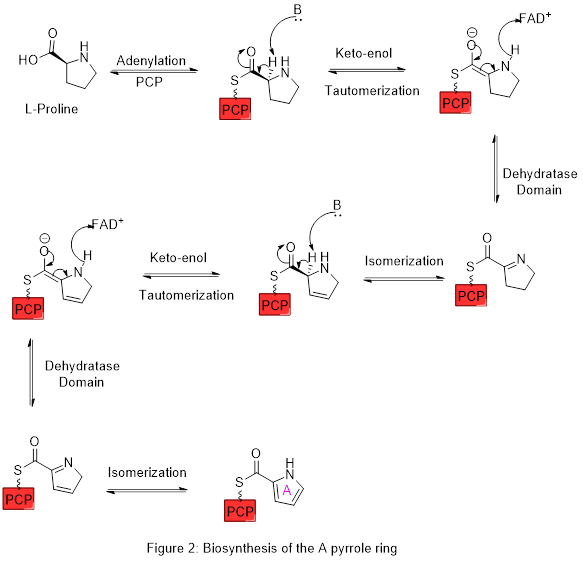
Ring A is synthesized from L-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD+ to yield pyrrole ring A.
The biosynthesis of Prodigiosin involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-serine, L-methionine, pyruvate, and 2-octenal.
Ring A is synthesized from L-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD+ to yield pyrrole ring A.
 Ring A is then expanded via the polyketide synthase pathway to incorporate L-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of L-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from L-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A–B ring structures.
Ring A is then expanded via the polyketide synthase pathway to incorporate L-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of L-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from L-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A–B ring structures.

Reactions and reactivity
Due to its aromatic character, pyrrole is difficult tohydrogenate
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
, does not easily react as a diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. ...
in Diels–Alder reactions, and does not undergo usual olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
reactions. Its reactivity is similar to that of benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
and aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aroma ...
, in that it is easy to alkylate and acylate. Under acidic conditions, pyrroles polymerize
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many for ...
easily, and thus many electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
reagents that are used in benzene chemistry are not applicable to pyrroles. In contrast, substituted pyrroles (including protected
Protection is any measure taken to guard a thing against damage caused by outside forces. Protection can be provided to physical objects, including organisms, to systems, and to intangible things like civil and political rights. Although th ...
pyrroles) have been used in a broad range of transformations.
Reaction of pyrrole with electrophiles
Pyrroles generally react with electrophiles at the α position (C2 or C5), due to the highest degree of stability of the protonated intermediate. Pyrroles react easily with
Pyrroles react easily with nitrating
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and ...
(e.g. HNO3/ Ac2O), sulfonating ( Py·SO3), and halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
ating (e.g. NCS, NBS, Br2, SO2Cl2, and KI/ H2O2) agents. Halogenation generally provides polyhalogenated pyrroles, but monohalogenation can be performed. As is typical for electrophilic additions to pyrroles, halogenation generally occurs at the 2-position, but can also occur at the 3-position by silation of the nitrogen. This is a useful method for further functionalization of the generally less reactive 3-position.
Acylation
Acylation
In chemistry, acylation (or alkanoylation) is the chemical reaction in which an acyl group () is added to a compound. The compound providing the acyl group is called the acylating agent.
Because they form a strong electrophile when treated with ...
generally occurs at the 2-position, through the use of various methods. Acylation with acid anhydride An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid.
In organic chemistry, organic acid anhydrides contain the functional group R(CO)O(CO)R'. Organic acid anhydrides often form when one equivalent ...
s and acid chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
s can occur without a catalyst; alternatively, a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
may be used. 2-Acylpyrroles are also obtained from reaction with nitriles, by the Houben–Hoesch reaction
The Hoesch reaction or Houben–Hoesch reaction is an organic reaction in which a nitrile reacts with an arene compound to form an aryl ketone. The reaction is a type of Friedel-Crafts acylation with hydrogen chloride and a Lewis acid catalyst.
Th ...
. Pyrrole aldehydes can be formed by a Vilsmeier–Haack reaction
The Vilsmeier–Haack reaction (also called the Vilsmeier reaction) is the chemical reaction of a substituted amide (1) with phosphorus oxychloride and an electron-rich arene (3) to produce an aryl aldehyde or ketone (5). The reaction is named af ...
. ''N''-Acylation of simple pyrrole does not occur.

Alkylation
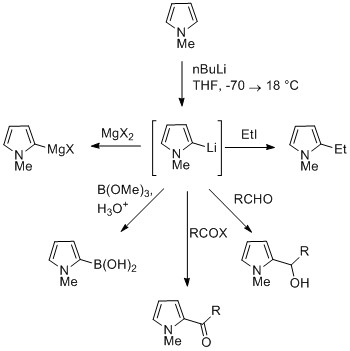
Electrophilic alkylation of simple pyrrole is uncommon. Alkylation to form enones at C2 has been seen.Reaction of deprotonated pyrrole
The NH proton in pyrroles is moderately acidic with a p''K''a of 16.5. Pyrrole can be deprotonated with strong bases such asbutyllithium Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis:
* ''n''-Butyllithium, abbreviated BuLi or nBuLi
* ''sec''-Butyllithium, abbreviated ''sec''-BuLi or sBuLi, has 2 stereoisomers, ...
and sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in co ...
. The resulting alkali pyrrolide is nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. Treating this conjugate base with an electrophile such as iodomethane
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one h ...
gives ''N''-methylpyrrole. ''N''-Metalated pyrrole can react with electrophiles at the N or C positions, depending on the coordinating metal. More ionic nitrogen–metal bonds (such as with lithium, sodium, and potassium) and more solvating solvents lead to ''N''-alkylation. Nitrophilic metals, such as MgX, lead to alkylation at C (mainly C2), due to a higher degree of coordination to the nitrogen atom.
In the cases of ''N''-substituted pyrroles, metalation of the carbons is more facile. Alkyl groups can be introduced as electrophiles, or by cross-coupling reactions.
 Substitution at C3 can be achieved through the use of ''N''-substituted 3-bromopyrrole, which can be synthesized by bromination of ''N''-silylpyrrole with NBS.
Substitution at C3 can be achieved through the use of ''N''-substituted 3-bromopyrrole, which can be synthesized by bromination of ''N''-silylpyrrole with NBS.
Reductions
Pyrroles can undergo reductions topyrrolidines
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most ...
and to pyrrolines. For example, Birch reduction
The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent (traditionally ...
of pyrrole esters and amides produced pyrrolines, with the regioselectivity depending on the position of the electron-withdrawing group.
Cyclization reactions
Pyrroles with ''N''-substitution can undergocycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
reactions such as +2, +2, and +1cyclizations. Diels-Alder cyclizations can occur with the pyrrole acting as a diene, especially in the presence of an electron-withdrawing group on the nitrogen. Vinylpyrroles can also act as dienes.
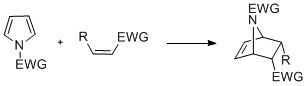 Pyrroles can react with
Pyrroles can react with carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s, such as dichlorocarbene
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly ...
, in a +1cycloaddition. With dichlorocarbene
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly ...
, a dichlorocyclopropane intermediate is formed, which breaks down to form 3-chloropyridine (the Ciamician–Dennstedt rearrangement).

Commercial uses
Polypyrrole is of some commercial value. ''N''-Methylpyrrole is a precursor to ''N''-methylpyrrolecarboxylic acid, a building-block in pharmaceutical chemistry. Pyrroles are also found in several drugs, includingatorvastatin
Atorvastatin is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a first-line treatment. It is taken by mouth.
Common ...
, ketorolac
Ketorolac, sold under the brand names Toradol, and Biorolac among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain. Specifically it is recommended for moderate to severe pain. Recommended duration of treatment is less ...
, and sunitinib
Sunitinib, sold under the brand name Sutent, is a medication used to treat cancer. It is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and i ...
. Pyrroles are used as lightfast red, scarlet, and carmine pigments.
Analogs and derivatives
Structural analog
A structural analog (analogue in modern traditional English; Commonwealth English), also known as a chemical analog or simply an analog, is a compound having a structure similar to that of another compound, but differing from it in respect to a ce ...
s of pyrrole include:
* Pyrroline Pyrrolines, also known under the name dihydropyrroles, are three different heterocyclic organic chemical compounds that differ in the position of the double bond. Pyrrolines are formally derived from the aromate pyrrole by hydrogenation. 1-Pyrrolin ...
, a partially saturated analog with one double bond
* Pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most ...
, the saturated hydrogenated analog
Derivatives of pyrrole include indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environmen ...
, a derivative with a fused benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
ring.
See also
*Simple aromatic ring
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated planar ring system. Many simple aromatic rings have trivial names. They are usually found as substructures of ...
s
* Tetrapyrrole
Tetrapyrroles are a class of chemical compounds that contain four pyrrole or pyrrole-like rings. The pyrrole/pyrrole derivatives are linked by ( =- or -- units), in either a linear or a cyclic fashion. Pyrroles are a five-atom ring with four car ...
* Polypyrrole
Polypyrrole (PPy) is an organic polymer obtained by oxidative polymerization of pyrrole. It is a solid with the formula H(C4H2NH)nH. It is an intrinsically conducting polymer, used in electronics, optical, biological and medical fields.
History ...
* Azonine
References
Further reading
* *External links
Synthesis of pyrroles (overview of recent methods)
Substitution reaction mechanisms of nitrogen-containing heteroaromatics
{{Authority control